Abstract
Introduction: The Phase 3 GENESIS trial (NCT03246529) compared the efficacy and safety of motixafortide and granulocyte-colony stimulating factor (M+G) to placebo + G (PBO+G) for the mobilization of hematopoietic stem cells (HSCs) prior to autologous stem cell transplantation (ASCT) in patients with multiple myeloma (MM). Compared with PBO+G, M+G significantly increased the proportion of patients who were able to successfully mobilize ≥6x106 CD34+ cells/kg for ASCT within 2 apheresis days (92.5% vs. 26.2%; p<0.0001). In a cost-effectiveness model (CEM) based on the GENESIS trial, M+G yielded additional quality-adjusted life years (QALYs) and cost savings relative to G alone from a United States (US) healthcare payer perspective (Lamotte et al. (2022) Value Health 25(7): S346). Plerixafor + G (P+G) is also used for HSC mobilization prior to ASCT in patients with MM. To date, M+G and P+G have not been directly compared in head-to-head trials; therefore, the relative efficacy and cost effectiveness of these therapies is currently unknown.
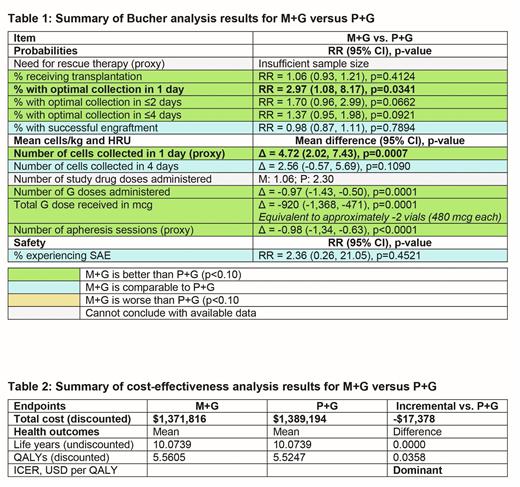
Methods: The Bucher method was used to assess the relative efficacy of M+G in the GENESIS trial and P+G in the Phase 3 AMD-3102 trial (NCT00103662), with the PBO+G arms of both trials used as a common comparator. Risk ratios (RRs) were derived for binary trial endpoints (i.e., proportion with mobilization of ≥6x106 CD34+ cells/kg within 1-4 apheresis days, successful engraftment, use of rescue therapy, proportion who underwent ASCT, incidence of serious adverse events [SAEs]) and mean differences were derived for continuous trial endpoints (i.e., number of study drug doses received, frequency and amount of G doses, and number of apheresis sessions). The RRs were then used to update the CEM to compare the cost effectiveness of M+G and P+G from a US healthcare payer perspective over a lifetime horizon; model assumptions and sources for unit costs and quality of life scores were unchanged from the previously published CEM.
Results: The mean age at baseline was 59-60 years in GENESIS and 58 years in AMD-3102; heterogeneity was observed for the mean time since diagnosis (6-7 vs. 11-12 months), prior radiotherapy (10%-11% vs. 27%-31%), and the ethnic and sex distribution of the PBO+G arms (White: 95% vs. 83%; males: 57% vs. 70%). Notably, prior lenalidomide use was markedly lower in AMD-3102 (<10%) than in GENESIS (70%), which likely contributed to the higher mobilization rates in the AMD-3102 PBO+G arm than in the GENESIS PBO+G arm. In the Bucher analysis, healthcare resource utilization (HRU) outcomes significantly favored M+G during primary mobilization, including less frequent administration of G (mean difference [MD]: -0.97; 95% CI -1.43, -0.50; p=0.0001), lower G doses (MD: -920 mcg; 95% CI -1,368, -471; p=0.0001), and fewer apheresis sessions (MD: -0.98; 95% CI -1.34, -0.63; p<0.0001) (Table 1). The proportion of patients with successful mobilization of ≥6x106 CD34+ cells/kg within 1 apheresis day was significantly greater with M+G (88.8%) than with P+G (54.2%; RR 2.97; 95% CI 1.08, 8.17; p=0.0341). Similar results were observed between groups for other outcomes, including ASCT rates (RR 1.06; 95% CI 0.93, 1.21; p=0.4124), engraftment durability by 100 days (RR 0.98; 95% CI 0.87, 1.11; p=0.7894), and SAE rate (RR 2.36; 95% CI 0.26, 21.05; p=0.4521); analyses were limited to publicly available data for P+G, and were not feasible for the use of rescue therapy because of insufficient sample size. In the cost-effectiveness analysis, M+G was dominant to P+G over a lifetime horizon, resulting in additional QALYs (+0.0358) and lower total costs (-$17,378 USD; Table 2) as per base case deterministic results. The key cost drivers were the probability of successful transplantation, long-term maintenance costs, and the time to engraftment in poor mobilizers. In a probabilistic sensitivity analysis, M+G was dominant in 66% of simulations and 92% cost effective at a WTP threshold of $50,000 per QALY.
Conclusions: The results of this analysis show that the use of M+G was associated with significantly lower HRU relative to P+G for the mobilization of HSCs prior to ASCT in patients with MM, as well as significantly greater successful mobilization of ≥6x106 CD34+ cells/kg within 1 apheresis day. Cost-effectiveness analysis results showed that M+G was dominant to P+G, yielding additional QALYs and net cost savings over a lifetime horizon from a US healthcare payer perspective.
Disclosures
Lamotte:BioLineRx, Ltd.: Consultancy; IQVIA: Current Employment; J&J: Other: Spouse's employer. DiPersio:VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; WUGEN: Current equity holder in private company, Research Funding; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; BioLineRx, Ltd.: Research Funding; Macrogenics: Research Funding; NeoImmune Tech: Research Funding; Amphivena Therapeutics: Research Funding; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Siegel:BMS: Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau; Takeda: Honoraria; Celularity: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Merck: Honoraria; COTA: Current equity holder in private company, Current holder of stock options in a privately-held company. Meron:BioLineRx, Ltd.: Current Employment. Gerlier:BioLineRx, Ltd.: Consultancy; IQVIA: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal